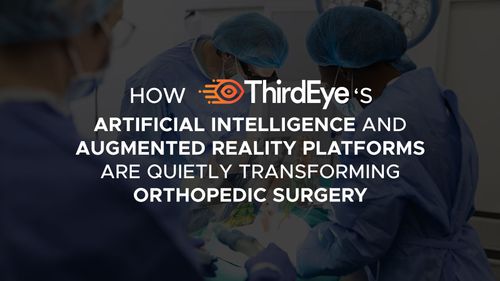
Surgery Shouldn’t Require Looking Away
February 17, 2026Every time a surgeon turns their head to check a monitor, something subtle happens.
Attention splits.
Flow breaks.
Milliseconds pass.
In most modern operating rooms, the most critical information — imaging, vitals, navigation guidance — still lives outside the surgeon’s direct field of view. Surgeons operate in one physical space while essential data exists in another.
That separation may seem small. But over the course of a complex procedure, it introduces constant micro-disruptions.
This is the hidden inefficiency in advanced medicine.
ThirdEye MedTech is rebuilding surgery around a simple principle:
The operating room should adapt to the surgeon — not the other way around.
From Screens to Spatial Intelligence
Traditional surgical workflows rely on:
Wall-mounted imaging displays
Manual cross-referencing between CT/MRI scans and live anatomy
Verbal communication across a busy, high-noise OR
Memory-based procedural steps under pressure
Even in high-tech surgical suites, surgeons often have to mentally stitch together multiple streams of information while maintaining precision and sterility.
They glance at a monitor.
They reinterpret a 2D image.
They mentally project that information back onto a 3D patient.
It works — but it adds cognitive load.
Reimagining the Surgical Interface
ThirdEye’s AR + AI platform changes the architecture of the operating room.
Instead of:
Looking away from the operative field
Translating 2D scans into mental 3D models
Breaking focus to verify imaging or vitals
Surgeons can experience:
Imaging spatially aligned directly to the patient
Real-time AI guidance embedded in their line of sight
Dynamic alerts without disrupting sterility
Remote expert annotations anchored precisely to anatomy
This is not simply adding another screen.
It is transforming surgical information into context-aware, spatial intelligence — delivered exactly where decisions are being made.
Keeping Focus Where It Matters
Surgery demands precision, flow, and sustained concentration. When critical data becomes part of the surgeon’s natural field of vision, cognitive friction decreases.
Attention stays anchored.
Decision-making accelerates.
The operative field remains the center of awareness.
In high-stakes environments, eliminating even small inefficiencies can meaningfully improve performance.
Designing the OR Around the Surgeon
The future of surgery is not about more equipment — it’s about smarter integration.
By merging augmented reality, real-time AI analytics, and secure connectivity, ThirdEye Gen MedTech is reshaping how surgical information is delivered and experienced.
Because surgery shouldn’t require looking away.
It should provide the right insight, in the right place, at the right moment — seamlessly.
And when information aligns with vision, precision follows.
Collaboration & Partnership Opportunities
ThirdEye MedTech is actively advancing its development phase and welcomes strategic collaboration.
The company is seeking partnerships with:
Orthopedic hospitals and surgeons are interested in clinical evaluation
Universities and biomedical research institutions
Technology and manufacturing partners in sensor and wireless systems
Medical device OEMs and distributors are exploring co-development or integration
Innovation in healthcare accelerates through collaboration. ThirdEye MedTech invites forward-thinking partners who share the vision of intelligent, data-guided recovery.
About ThirdEyeGen
ThirdEyeGen has developed a comprehensive AR/MR digital ecosystem that combines proprietary hardware with AI-powered software, optimized for government and enterprise deployment. The company is led by a senior team with over 25 years of experience delivering next-generation defense products.
With a proven track record of deploying mission-critical solutions, ThirdEyeGen is gaining traction across defense, aerospace, medical, telecom, automotive, construction, and energy sectors — positioning it for continued expansion in high-growth global markets.
For partnership or collaboration inquiries:
Email: sales@thirdeyegen.com
Learn more: www.thirdeyegen.com